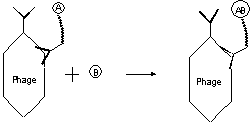Selection by reaction: a
general strategy to develop novel enzymes and catalysts and for functional
cloning
By Tianxin Wang, wtx@wtxtech.com
Controlling and catalyzing reaction like
enzymes is always the central topic of chemistry. In 1986, Lerner RA¡¯s group [1]
and Schultz PG¡¯s group [2] proposed the catalytic antibody strategy (abzyme),
which allow chemist to develop novel artificial enzymes to certain reactions,
which was a breakthrough in this field. Catalytic antibody is antibody induced
by synthetic transitional state mimics, it may stabilize the real transitional
state therefore catalyze the reaction. However, catalytic antibody strategy has
its intrinsic limitations, which greatly hindered its industrial
application:
1)
Only use
antibody as scaffold, limited structure diversity and therefore limited
functions.
2)
Hard to
utilize coenzyme and subunit.
3)
The good
transitional state mimics are not easy to design and synthesize, and even a good
transitional state mimics cannot exactly mimic the real transitional
state.
4)
Need to know the clear reaction mechanism
while many reactions¡¯ mechanisms are not clear yet and there are other ways for
catalysis besides stabilizing transitional state.
5)
Most
importantly, most antibodies induced by transitional state mimics have only very
good binding but no catalytic activity at all.
Till
now only one catalytic antibody has been used in small industrial scale (and in
fact even this one is developed using reactive hapten [3], an ingenious
improvement on catalytic antibody by Janda KD instead of using transitional
state mimics).
In
1997, the author invented a new strategy: Selection by Reaction. It overcome the
limitations of catalytic antibodies and could work for most reactions
theoretically. The detail is: first use a phage displayed library such as a
phage displayed antibody library (not limited to antibody library, therefore
have greater scaffold diversity), then couple the substrate with the phage, if
there is a phage expressed certain antibody that can catalyze the substrate to
give reaction product, under suitable condition the substrate on this phage
would become product and we can pick this phage out by affinity column for the
product. A directed evolution strategy could be applied until the catalytic
activity is optimized. The cross catalysis between phages could be reduced by
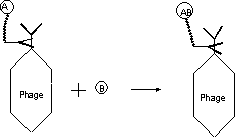
adjusting concentration, viscosity or immobilization. The following figure gives
a more detailed illustration, which use antibody specific to the surface protein
on phage or the constant region of antibody expressed to immobilize the
substrate instead of covalently coupling using chemicals, which may lack
specificity. For a reaction A+B¨¤ AB,
1)
|
|
Substrate-PEG-helper
antibody- constant region of catalytic antibody - phage
2)
|
|
Substrate-PEG-helper
antibody- other protein on the phage
Fig1, 2 catalytic antibodies on
phage catalyze substrate A which is immobilized on phage by the helper antibody
to give reaction product AB
This
method eliminate the need for design and synthesizing transitional state mimics
and need not to know the mechanism of the reaction, and could utilize other
catalyst scaffolds and other display system such as ribosome display library.
The author had proposed this strategy to the inventors of catalytic antibody
and received the reply from Dr. K. D. Janda. Several months later without
acknowledging my proposal to him, Schultz PG's group published a paper using a known
enzyme as model proved the validity of this strategy and suggested that it could
be used for protein functional cloning in proteomic study [4]. This method
overcomes the limitation of catalytic antibody and is showing [5, 6] great
potential in life science and chemistry.
This strategy is also not limited in searching novel protein enzymes, for example, it could also be used in developing small molecule catalysis on polymer support:
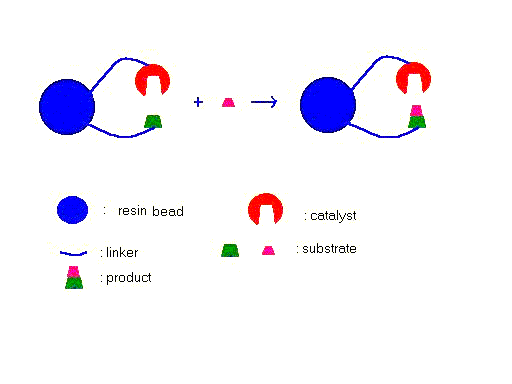
Fig.3 Catalyst on the resin bead
converts the substrate nearby into product for selection, a one bead one
compound library is used, antibody selective to product or isotope, dyes coupled
to the free substrate could be used to pick out the special bead having
catalytic activity .
1)
Tramontano
A, Janda KD, Lerner RA. Proc Natl
Acad Sci U S A 1986 September;83(18):6736-40; Science 1986 Dec
19;234(4783):1566-70
2)
Pollack SJ,
Jacobs JW, Schultz PG; Science 1986
December 19; 234 (4783):1570-3
3)
Janda KD,
Lo LC, Lo CH, Sim MM, Wang R, Wong CH, Lerner RA. Science 1997 February
14;275(5302):945-8
4)
Henrik
Pedersen , Holder S, Sutherlin DP, Schwitter U, King DS,
Schultz PG. Proc. Natl. Acad. Sci. USA. 1998
September 1; 95 (18): 10523¨C10528
5)
Liu, D.R.;
Schultz, P.G. Angew. Chem. Int. Engl. Ed., 38:36-54, 1999.
6)
Xia G, Chen
L, Sera T, Fa M, Schultz PG, Romesberg FE. Proc Natl Acad Sci U S A 2002 May
14;99(10):6597-602